Importance of assessing blood involvement
Blood class is integral to staging: To determine a patient’s clinical stage, levels of involvement in 4 compartments are assessed: skin, lymph node, viscera, and blood. This is referred to as the TNMB classification.1
Blood involvement must be assessed for accurate diagnosis, staging, and optimal patient management.1
B classification
Because blood involvement is an independent prognostic factor in Mycosis Fungoides and Sézary Syndrome, it is important to determine the baseline level at diagnosis—classified as B0-B21,2:
- B0: No blood involvement
- B1: Low blood involvement—may occur at any stage (except for IIIA)
- B2: High blood involvement—advanced disease, regardless of compartment involvement
Blood can be involved at early stages
Blood involvement is always present in Sézary Syndrome, but it can occur even at early stages of Mycosis Fungoides.1,3 Low-level blood involvement is associated with inferior survival, even in early-stage Mycosis Fungoides.4
1 in 5 patients
with early-stage (IA-IIA) Mycosis Fungoides
have B1
blood involvement3
Based on a PROCLIPI database study that analyzed hematological data from 348 patients with early-stage Mycosis Fungoides.3
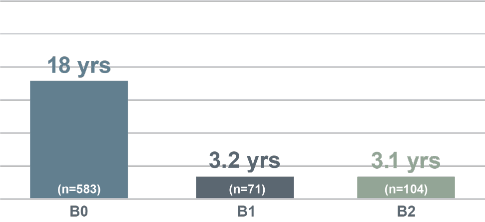
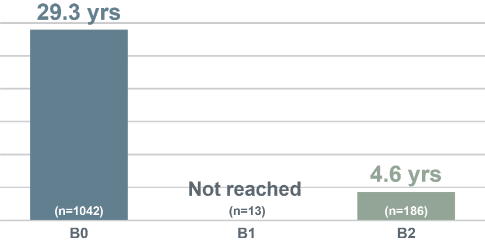
Blood involvement is associated with shorter survival5,6
- With increasing blood involvement, overall survival and disease-specific survival are reduced5-7
- One study found that, in patients with B2 blood involvement, there is a 4.6x greater risk of progression5
The full significance of B1 level blood involvement remains the subject of further research.8
Median Overall Survival (Years) by Blood Classification, Agar et al (n=1502)5
Median Overall Survival (Years) by Blood Classification, Talpur et al (N=1263)6

Assessing blood with flow cytometry
Flow cytometry in CTCL
Flow cytometry is the preferred method for measuring blood involvement in Mycosis Fungoides and Sézary Syndrome.1 It
analyzes cells by measuring light scatter of fluorescently labeled cells.9
The flow cytometry process9
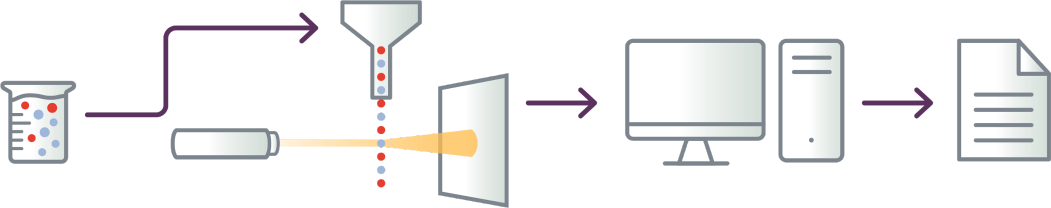
- Cells are tagged with fluorescent markers of interest and suspended in solution
- Fluorescent cells are injected into the flow cytometer and evaluated as they flow past multiple lasers
- Each cell is analyzed for visible light scatter and multiple fluorescence parameters
- Light scatter information is collected and processed by a computer
- Computer-generated output provides basis for diagnosis; report contains data on
abnormal T-cell populations

- Cells are tagged with fluorescent markers of interest and suspended in solution
- Fluorescent cells are injected into the flow cytometer and evaluated as they flow past multiple lasers
- Each cell is analyzed for visible light scatter and multiple fluorescence parameters

- Light scatter information is collected and processed by a computer

- Computer-generated output provides basis for diagnosis; report contains data on abnormal T-cell populations
It provides qualitative and quantitative measures of abnormal T cells in blood (eg, immunophenotype,
T cell ratios).11,12
NCCN, EORTC, and International Society for Cutaneous
Lymphomas (ISCL)
recommend the
use of flow cytometry
for evaluation
of CTCL
stage1,10
When to consider flow cytometry
Recently published consensus guidance from an international group of cytometry experts recommend flow cytometry at
diagnosis and throughout treatment when clinical flags appear.2
Flow cytometry can aid in:
- Accurate diagnosis, staging, and informing prognosis
- Establishing baseline for monitoring changes in blood burden over time
- Guiding treatment approach
flags appear
- In the case of disease/stage progression
- Patients with advanced disease (stage IIB and above)
- Intractable pruritus
- Generalized patches and/or plaques (T2A/T2B)
- Erythroderma
- Lymphocytosis on WBC
- High serum LDH
- Lack of response to treatment
How often to use flow cytometry
Flow cytometry may be considered during follow-up2:
- Every 3 months in patients with abnormal flow cytometry at baseline
- Upon development of any clinical flags
Recommended flow cytometry panel for CTCL
International guidelines recommend a minimum of 6 panel markers.11
No single T-cell marker alone can accurately identify Sézary cells. Diagnosis is highly dependent on evaluation of multiple T-cell markers combined in a single-tube flow cytometry assay.11
Recommended flow cytometry panel11
| Identification of leukocytes and T cells |
|
|---|---|
| CD45 | Identifies all leukocytes |
| CD3 | Identifies all T cells |
| CD4, CD8 | Identifies T cell subsets Ratio of CD4:CD8 can provide additional important information |
| Detection of Sézary cells | |
|---|---|
| CD7, CD26 | Frequently absent in Mycosis Fungoides and Sézary Syndrome |
Loss of CD7 and/or CD26 are common T-cell abnormalities in Mycosis Fungoides and Sézary Syndrome11:
- Loss of CD7 is identified in 50% to 80% of cases with blood involvement
- Loss of CD26 is identified in 70% to 100% of cases with blood involvement
Mycosis Fungoides or
Sézary Syndrome10

How to order flow cytometry
Important information when ordering flow cytometry
Specify diagnosis in request
Specify that workup for Mycosis Fungoides and/or Sézary Syndrome is needed in the flow request form (eg, rule out Mycosis Fungoides, or workup for suspected Sézary Syndrome). This helps the pathologist to choose the disease-focused panel with the appropriate markers for detecting Sézary cells (eg, CD26). CD4+/CD7- and/or CD4+/CD26- are common phenotypes seen in Mycosis Fungoides and Sézary Syndrome.1,11
Confirm where to send the specimen
At least a 6-color flow cytometry test is needed to identify the malignant Sézary cells.12 However, not all flow cytometry labs have this capability. Call lab to confirm where to send the specimen.
Include recent CBC results
Submit recent CBC results to determine absolute number of aberrant lymphocytes.1 The absolute value is determined by the percentage of aberrant lymphocytes identified on flow cytometry multiplied by the total lymphocyte count and can be calculated as shown below1:
- Absolute Sézary count = Total lymphocyte count x percentage of atypical T-cell population
- Example calculation: 3.36 K/µl x 0.62 = 2.083 K/µl or 2083 cells/µl
Use the same flow center
Send samples to the same flow center for sequential testing to help ensure consistency in flow methodology and the report summary.1,12
Consider consultation
Consultation with CTCL specialists is recommended for diagnosis and staging, and for optimal patient management.10
Lab-specific instructions
Below is specific information about labs that conduct flow cytometry for CTCL, including the name of the panel, test code, and biomarkers that are included or available, as well as additional instructions for ordering. This information is provided for your knowledge. Kyowa Kirin does not endorse any of the labs listed below.
Test Name
Leukemia/Lymphoma Evaluation
Lab Test Code
- 35080
- For New York patients: 37340
Biomarkers Included/Available
- Initial markers evaluated: CD2, CD3, CD4, CD5, CD7, CD8, CD10, CD11c, CD13, CD19, CD20, CD23, CD33, CD34, CD38, CD56,
CD64, CD117, HLA‐DR, sKappa, sLambda- CD45 is used for gating
Additional markers may be performed based on the pathologist review.
Lab Instructions
- CD26 may not automatically be included in the Leukemia/
Lymphoma screening panel - Indicate Mycosis Fungoides/
Sézary Syndrome diagnosis for the lab to include Sézary markers (CD26) and report out on CD4+/CD7- and CD4+/CD26- cells - Call lab to confirm where to send the specimen
For questions, call 866-697-8378
Test Name
Leukemia/Lymphoma Phenotyping, Flow Cytometry
Lab Test Code
- 480260
Biomarkers Included/Available
- Markers included: CD2, CD3, CD4, CD5, CD7, CD8, CD10, CD11c, CD13, CD16, CD20, CD22, CD23, CD38, CD56, CD57, CD103, FMC-7, Kappa, Lambda
- CD45 is used for gating
Lab Instructions
- CD26 may not automatically be included in the Leukemia/Lymphoma screening panel
- Indicate Mycosis Fungoides/
Sézary Syndrome diagnosis for the lab to include Sézary markers (CD26) and report out on CD4+/ CD7- and CD4+/CD26- cells - Call lab to confirm where to send the specimen
For questions, call 800-345-4363
Test Name
Sézary Diagnostic Flow Cytometry, Blood
Lab Test Code
- SZDIA, SZMON
Biomarkers Included/Available
- Triage Panel: CD3, CD16
- Sézary Panel: CD2, CD4, CD5, CD7, CD8, CD26, and TRBC1
Lab Instructions
- This Sézary panel is ordered for patients with a clinical suspicion of Sézary Syndrome or cutaneous T-cell lymphoma with peripheral blood involvement without a previously confirmed diagnosis. A Triage panel and Sézary panel will always be performed. This test is not indicated for monitoring peripheral blood involvement in patients with a diagnosis of Sézary Syndrome or Mycosis Fungoides. For monitoring purposes, order SZMON/Sézary Monitoring Flow Cytometry, Blood.
For questions, call 800-533-1710
Learn more about the test at the Mayo Clinic Laboratories website
Test Name
Leukemia/Lymphoma Phenotyping Evaluation by Flow Cytometry
Lab Test Code
- 3001780
Biomarkers Included/Available
- T cell: CD1a, CD2, CD3, CD4, CD5, CD7, CD8, TCR alpha-beta, CD10, CD25, CD26, CD30, CD200, CD279 (PD-1), TCR gamma-delta, TRBC1, Cytoplasmic CD3
- B cell: CD5, CD10, CD11c, CD19, CD20, CD22, CD23, CD25, CD38, CD103, CD123, CD200, surface Kappa, surface Lambda, cytoplasmic Kappa, cytoplasmic Lambda
- Plasma cell: CD13, CD19, CD20, CD27, CD33, CD38, CD45, CD56, CD81, CD117, CD138, CD200, cytoplasmic Kappa, cytoplasmic Lambda
- Myeloid: CD11b, CD13, CD14, CD15, CD16, CD33, CD34, CD36, CD38, CD41, CD42b, CD45, CD56, CD57, CD61, CD64, CD66b, CD71, CD117, CD123, HLA-DR, myeloperoxidase, glycophorin (CD235a), TdT
- B lymphoblasts: CD10, CD19, CD20, CD22, CD24, CD38, CD45, CD58, CLRF2, cCD22, cCD79a
- Mast cell: CD2, CD25, CD30, CD117, CD123
- Requests for specific markers to be run must be listed on manual requisition or by footnote for electronic orders
- We do not offer individual marker identification separately outside of the markers in this panel
- The report will include a pathologist interpretation and a marker interpretation range corresponding to CPT codes
of 2-8 markers, 9-15 markers, and 16+ markers interpreted. Charges apply per marker - International guidelines recommend a minimum of 6 panel markers to identify malignant Sézary cells: CD3, CD4, CD8, CD7, CD26, CD45
Not all markers will be reported in all cases.
Lab Instructions
For questions, call 800-522-2787
Test Name
TRBC1/T-Cell Lymphoma Companion Panel
Lab Test Code
- n/a
Biomarkers Included/Available
- Available Markers: CD3, CD4, CD7, CD8, CD25, CD26, CD30, CD45, CD279, and TRBC1
Not all markers will be reported in all cases.
Lab Instructions
- Available as stand-alone test (as described here) or as add-on to panels
For questions, call 866-776-5907, Option 3
Test Name
Specialized Hematopathology Laboratory
Lab Test Code
- n/a
Biomarkers Included/Available
- A comprehensive list of markers are available for flow cytometry testing, including CD3, CD4, CD7, CD8, CD26, CD45
Lab Instructions
- Offers ten-color, 15-antibody panel for high-volume testing that can separate normal cell populations from residual disease. Disease-focused panels are offered
- Indicate Mycosis Fungoides/
Sézary Syndrome diagnosis on the request form
For questions, call 914-339-5000
Green indicates Sézary cell-specific markers.
This is not a complete list of labs that perform flow cytometry for Sézary cells.
For additions to the list, changes, or corrections, please contact USOncologyMarketing@kyowakirin.com
Updated in March 2025.



