What is flow cytometry?
Flow cytometry is a tool that analyzes cells by measuring light scatter of fluorescently labeled cells.1
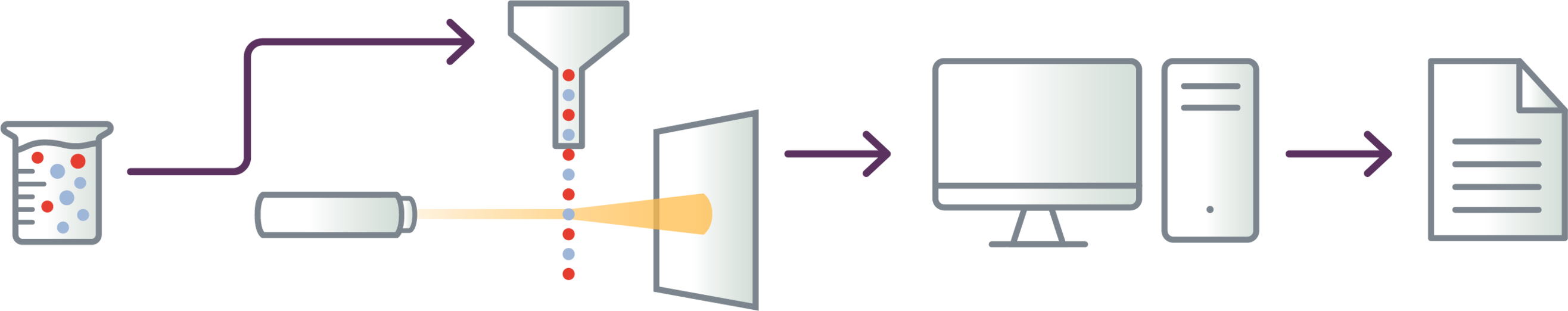

Cells are tagged with fluorescent markers of interest and suspended in solution
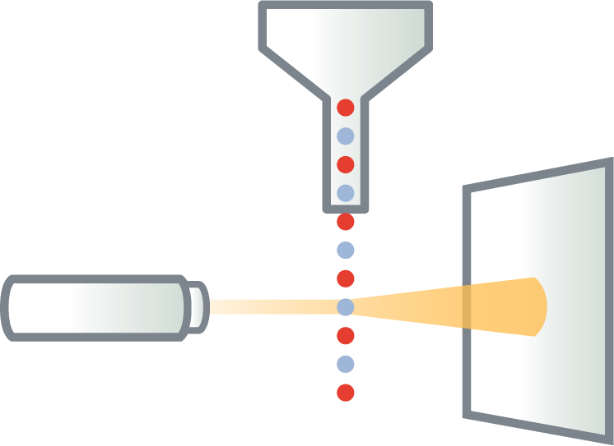
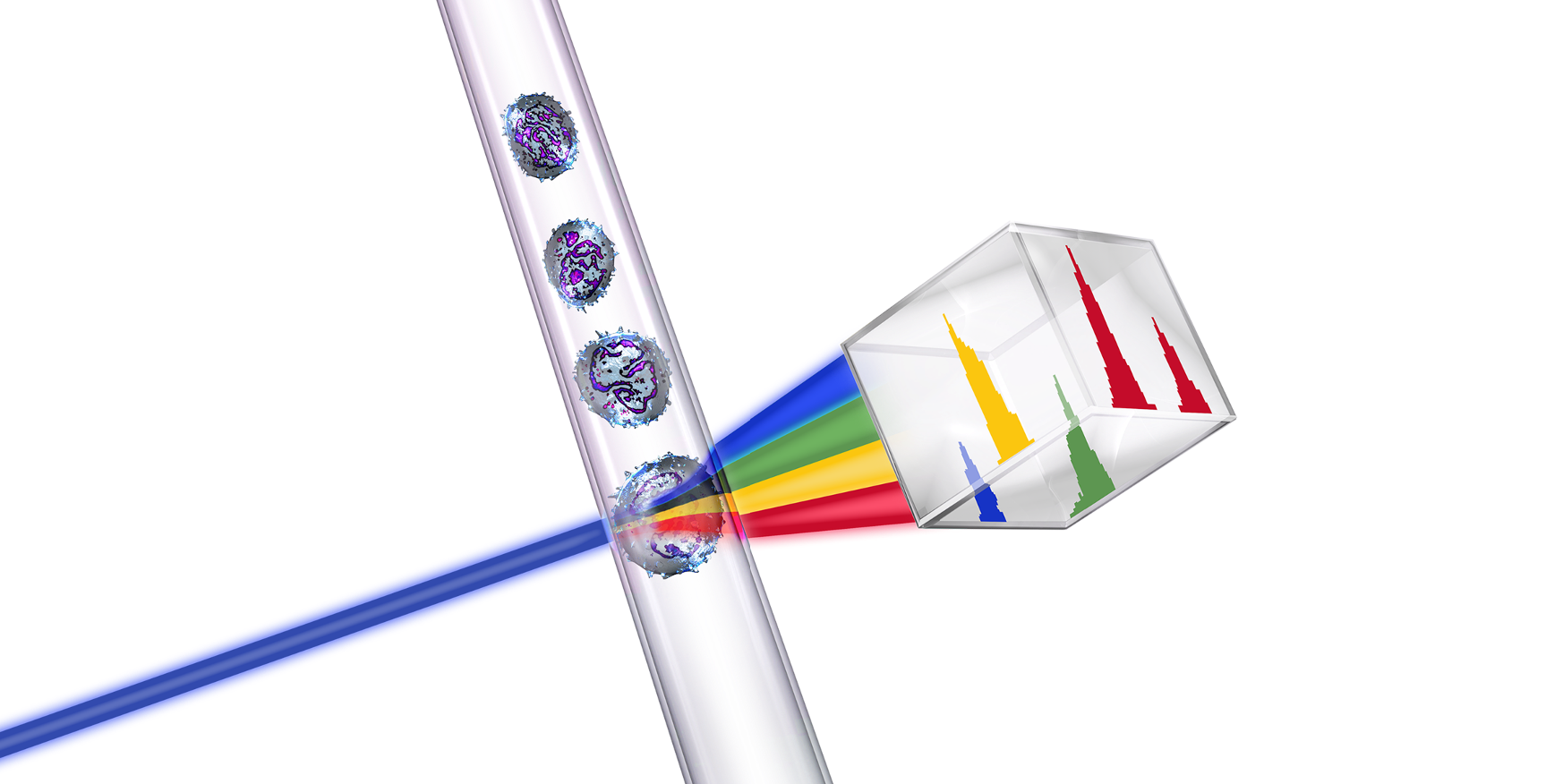
Fluorescent cells are injected into the flow cytometer and evaluated as they flow past multiple lasers
Each cell is analyzed for visible light scatter and multiple fluorescence parameters

Light scatter information is collected and processed by a computer

Computer-generated output provides basis for diagnosis; report contains data on abnormal T-cell populations
A reliable method for measuring abnormal T-cells in blood
Flow cytometry is the preferred method for measuring blood involvement in Mycosis Fungoides and Sézary Syndrome.2 It provides qualitative and quantitative measures of abnormal T-cells in blood (eg, immunophenotype, T-cell ratios).3,4
Robust5
Quantifiable6
Objective6
Consistent7
Increasingly used6
Technologically precise8-9
NCCN, EORTC, and International Guidelines for Cutaneous Lymphoma (ISCL) recommend the use of flow cytometry for evaluation of CTCL stage2,10
Until recently, the assessment of blood involvement was made using manual counts of morphologically atypical T-cells (Sézary cells). But this approach is subjective, time consuming, and affected by interobserver variability.6
Recommended flow panel for CTCL5
International guidelines recommend a minimum of 6 panel markers11
No single T-cell marker alone can accurately identify Sézary cells. Diagnosis is highly dependent on evaluation of multiple T-cell markers combined in a single-tube flow cytometry assay.11
| Identification of leukocytes and T-cells | ||
|---|---|---|
| CD45 | Identifies all leukocytes | |
| CD3 | Identifies all T-cells | |
| CD4, CD8 |
Identifies T-cell subsets Ratio of CD4:CD8 can provide additional important information |
|
| Detection of Sézary cells | |
|---|---|
| CD7, CD26 | Frequently absent in Mycosis Fungoides and Sézary Syndrome |
Loss of CD7 and/or CD26 are common T-cell abnormalities in Mycosis Fungoides and Sézary Syndrome. Loss of CD7 is identified in 50% to 80% and loss of CD26 is identified in 70% to 100% of cases with blood involvement.6,11
CD4+/CD7- and/or CD4+/CD26- are common phenotypes seen in Mycosis Fungoides and Sézary Syndrome5,11
When to consider flow cytometry
Recently published consensus guidance from an international group of cytometry experts recommend flow cytometry at diagnosis and throughout treatment when clinical flags appear. Flow cytometry may also be considered during follow-up.6
Consensus recommendations on when to consider flow cytometry6
At diagnosis
Flow cytometry can aid in:
- Accurate diagnosis, staging, and informing prognosis
- Establishing baseline for monitoring changes in blood burden over time
- Guiding treatment approach
When the following clinical flags appear
- In the case of disease/stage progression
- Patients with advanced disease (stage IIB and above)
- Intractable pruritus
- Generalized patches and/or plaques (T2A/T2B)
- Erythroderma
- Lymphocytosis on WBC
- High serum LDH
- Lack of response to treatment
How often during follow-up?7
- Every 3 months in patients with abnormal flow cytometry at baseline
- Upon development of any clinical flags
The flow report will provide an interpretation of the results with a clinical interpretation, and typically also includes a breakdown of percentages and quantities of lymphocyte types.3,8 Additional information included in the report may vary by institution.8,11
Key components included in a flow cytometry report3
Patient information and treatment history
Includes information provided by the HCP in the flow request.
- Patient ID
- Current diagnosis
- Current treatment(s)
- Clinical history
- Assay objective (eg, Suspicion of Mycosis Fungoides. Rule out CTCL)
Clinical interpretation of results (negative/positive)
Will appear in the beginning portion of the report, and will include interpretation of the results specifying whether the results were consistent with CTCL. B-classification may also be specified.
What may be included in the clinical interpretation3
- Presence or absence of abnormal T-cells based on visual interpretation of the flow data
- Description of the abnormal population if present, including phenotype
- Relative percentage of abnormal T-cells
Example of a negative flow result
No distinct population of abnormal T-cells is detected. No expanded CD4+ T-cell population is detected. CD4:CD8 ratio is 3:1. T-cells with CD4+/CD7- immunophenotype are 3.0% of all analyzed cells and 10.0% of lymphocytes, and have a heterogenous CD7 expression pattern. No other significant T-cell immunophenotypic abnormalities are detected.
Example of a positive flow result
Atypical T-cell population consistent with peripheral blood involvement. Immunophenotype: variable CD2, dim CD3, CD4+ and CD7-. T-cell gene rearrangement analysis: positive for clonal rearrangements in TCR-gamma and TCR-beta.
B2 classification + TCR clone
Quantitative T-cell panel
Absolute counts and percentages of T-cell tested. May include other information such as CD4:CD8 ratio. CD4+/CD7- and CD4+/CD26- results may be highlighted.
Quantitative T-cell panel results may be presented in table format:
Absolute counts
May include a column that lists the # cells/unit volume (eg, 4.30 K/µL)
% of total lymphocytes
This column would list the percentage of each marker expressed relative to the total T-cell population (eg, 71% of total lymphocytes)
CD3
cells/µL
%
CD4
cells/µL
%
CD7
cells/µL
%
CD8
cells/µL
%
CD45
cells/µL
%
CD4+/CD7-
cells/µL
%
CD4+/CD26-
cells/µL
%
Summary: Flow cytometry matters
Why
Mycosis Fungoides and Sézary Syndrome are lymphomas that present in skin12
Blood involvement can develop at any stage and is associated with shorter survival10,13,14
Blood must be assessed for accurate diagnosing and optimal patient management6,10
When
- At diagnosis for accurate staging, and guiding treatment approach6
- Every 3 months if there is blood involvement2
- Upon disease progression
- Advanced disease (stage IIB and above)
- Intractable pruritus
- Generalized patches/plaques (T2A/T2B)
- Erythroderma
- Abnormal lab results
- Extracutaneous disease
- Refractory to treatment
How
Using flow cytometry to measure a minimum of 6 panel markers; no single antigen by itself can accurately diagnose Mycosis Fungoides or Sézary Syndrome11
Recommended T-cell panel for Mycosis Fungoides and Sézary Syndrome11
CD3CD8
CD4CD26
CD7CD45
Watch expert perspectives on flow cytometry
Alejandro A. Gru, MD, Leonard C. Harber Professor of Dermatology and Director of Dermatopathology; Columbia University Irving Medical Center discusses why flow cytometry is the recommended method for assessing blood involvement in Mycosis Fungoides and Sézary Syndrome. Dr. Gru explores how flow cytometry works, recommended panel markers, the flow report, and when to consider flow cytometry.
References: 1. McKinnon KA. Flow cytometry: an overview. Curr Protoc Immunol. 2018;120:5.1.1-5.1.11. 2. Olsen EA, Whittaker S, Willemze R, et al. Primary cutaneous lymphoma: recommendations for clinical trial design and staging update from the ISCL, USCLC, and EORTC. Blood. 2022;140:419-437. 3. Illingworth A, Johansson U, Huang S, et al. International guidelines for the flow cytometric evaluation of peripheral blood for suspected Sézary syndrome or mycosis fungoides: assay development/optimization, validation, and ongoing quality monitors. Cytometry B Clin Cytom. 2021;100(2):156-182. 4. Craig F. It is time to adopt a multicolor immunophenotyping approach to evaluate blood for Sézary syndrome and mycosis fungoides. Cytometry B Clin Cytom. 2021;100(2):125-128. 5. Tembhare PR, Chatterjee G, Chaturvedi C, et al. Critical role of flow cytometric immunophenotyping in the diagnosis, subtyping, and staging of T/NK-cell non-Hodgkin’s lymphoma in real-world practice: A study of 232 cases from a tertiary cancer center in India. Front Onc. 2022;12:doi: 10.3389/fonc.2022.779230. 6. Vermeer MH, Moins-Teisserenc H, Bagot M, et al. Flow cytometry for the assessment of blood tumour burden in cutaneous T-cell lymphoma: towards a standardized approach. Br J Dermatol. 2022;187(1):21-28. 7. Vermeer MH, Nicolay JP, Scarisbrick JJ, Zinzani PL. The importance of assessing blood tumour burden in cutaneous T-cell lymphoma. Br J Dermatol. 2021;185(1):19-25. 8. Guitart J. Sézary syndrome and mycosis fungoides flow cytometric evaluation: the clinicians’ perspective. Cytometry B Clin Cytom. 2021;100 (2):129-131. 9. Craig FE, Foon KA. Flow cytometric immunophenotyping for hematologic neoplasms. Blood. 2008; 111(8):3941-3967. 10. Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Primary Cutaneous Lymphomas V.1.2023. © National Comprehensive Cancer Network, Inc. 2023. All rights reserved. Accessed March 5, 2023. To view the most recent and complete version of the guideline, go online to NCCN.org. 11. Horna P, et al. Flow cytometric evaluation of peripheral blood for suspected Sézary syndrome or mycosis fungoides: international guidelines for assay characteristics. Cytometry B Clin Cytom. 2021;100(2):142-155. 12. Willemze R, Cerroni L, Kempf W, et al. The 2018 update of the WHO-EORTC classification for primary cutaneous lymphomas. Blood. 2019;133(16):1703-1714. 13. Agar NS, Wedgeworth E, Crichton S, et al. Survival outcomes and prognostic factors in mycosis fungoides/Sézary syndrome: validation of the revised International Society for Cutaneous Lymphomas/European Organisation for Research and Treatment of Cancer staging proposal. J Clin Oncol. 2010;28(31):4730-4739. 14. Talpur R, Singh L, Daulat S, et al. Long-term outcomes of 1,263 patients with mycosis fungoides and Sézary syndrome from 1982 to 2009. Clin Cancer Res. 2012;18(18):5051-5060.

